On the eve of World Intellectual Property Day, the United States Trade Representative released its annual “Special 301 Report.” Developing countries like India and Malaysia once again face unfair pressure from the US government over the measures these countries have taken to try to protect access to medicines.
The Special 301 report is an annual report by USTR that names countries on “watch lists” in line with its powerful pharmaceutical industry’s[1] demands that call for more stringent intellectual property standards and enforcement in third world countries to undermine their competitors.
 As in past reports, this year’s Special 301 names countries making use of public health safeguards in intellectual property laws.
As in past reports, this year’s Special 301 names countries making use of public health safeguards in intellectual property laws.
Not surprisingly, India is named in the report for the country’s patentability criteria, compulsory licensing criteria and absence of an additional intellectual property monopoly-data exclusivity.
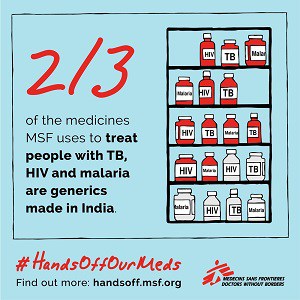
India’s patent and drug regulatory laws and policies have helped to protect price-lowering generic competition, so much so that the country is known as the “pharmacy of the developing world” because it supplies affordable quality generic medicines globally.
Such pressure violates the integrity and legitimacy of the system of legal rights and flexibilities created by the Trade-Related Aspects of Intellectual Property Rights (TRIPs) Agreement, as reaffirmed by the Doha Declaration for the World Trade Organisation members to meet their rights and public health obligations.
In this year’s report, USTR also extended its Out-of-Cycle Review of Malaysia and named the country for compulsory licensing “concerns.”

Doctors Without Borders/Médecins Sans Frontières (MSF) urges the Malaysian government to continue to reject any pressure from pharmaceutical corporations or their political allies to reverse the government use licence issued to enable access to an affordable version of the hepatitis C drug sofosbuvir.
MSF considers compulsory licensing a much needed and legally permissible tool to overcome patent barriers where necessary.
At a time when medicine prices are soaring — including in the U.S., the USTR’s report undermines the efforts of U.S. lawmakers and patient advocates seeking to make medicines more affordable domestically.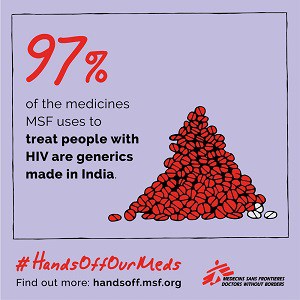
USTR’s push for more protection and enforcement of IP policies would keep medicine prices high globally and place lifesaving treatments out of reach for longer in developing countries, like those in which MSF works.
These unwarranted pressure tactics, if successful, shall limit countries’ scope of using internationally agreed public health safeguards, and threaten to undermine their domestic health efforts.
People all across the globe can’t afford their medicines because pharmaceutical corporations choose to charge unaffordable prices. Considering that people’s health and access to medicines is at stake, developing countries should not capitulate to United States’ pressure at the behest of multinational pharmaceutical corporations against the interest of all people who need access to medicines.
[1] Pharmaceutical Research and Manufacturers of America (PhRMA) and Biotechnology Innovation Organization (BIO) are lobby groups which represent the US pharmaceutical and Biologic drug industries.











