Stobdan Kalon is a medical doctor working with Medecins Sans Frontieres/Doctors Without Borders (MSF). His experience spans from managing infectious diseases, interventions in Drug Resistant-Tuberculosis (DR-TB), hepatitis and HIV.
As a doctor, I am elated that the World Health Organization (WHO) has updated their DR-TB treatment guidelines, which approves concomitant use of Bedaquiline and Delamanid and its use for longer than six months, as safe and tolerant for patients. It will help provide life saving treatment to hundreds of thousands of DR-TB patients around the world. It gives a greater sense of happiness as those revisions are based on emerging evidence and information, including findings from MSFs projects in Mumbai, India, where we have till now treated 256 patients with these life saving treatment regimens. Huge credit for this success story goes to patients themselves, some of whom have been champions for the access to life saving treatments. One such patient who stands out for her fight against TB and also helped speed up the access to Bedaquiline in India is a brave young girl called Shreya Tripathy.
When I first heard about Shreya in December 2016, she was fighting for her life and I received request from her father conveyed by our project team, to provide her treatment at MSF Mumbai clinic. This brave young girl from Bihar state had DR-TB and had already gone through the ordeal of multiple episodes of TB diagnosis and treatment with its delays, misdiagnosis and mistreatments which had now resulted in complicating her TB into the severest forms of drug resistant TB. At the time the new TB drugs- Bedaquiline and Delamanid, that would be a lifesaving option for many DR-TB patients like Shreya, wasn’t easily accessible to all as Bedaquiline was only available at the 6 pilot sites in India and Delamanid was only available through compassionate use (providing a drug still under investigation to patients, adhering to strict pre-approved criteria, without any other option). She travelled from Bihar, about 898 miles, to the opposite side of the subcontinent trying to find treatment. She finally arrived at the MSF project in Mumbai. We were able to support her treatment through our partnership with the Hinduja hospital.
Shreya’s case was covered widely in the media. She and her family fought a legal battle, demanding access to the new drug Bedaquiline. Their effort later helped speed up the roll-out of this treatment across the country. Unfortunately for Shreya, it was too late. Years of TB had taken a very heavy toll on her young body. After putting up a brave fight, she finally succumbed to DR-TB on October 09, 2018.
Stobdan Kalon
Medical Doctor, MSF
“Huge credit for this success story goes to patients themselves, some of whom have been champions for the access to life saving treatments.”
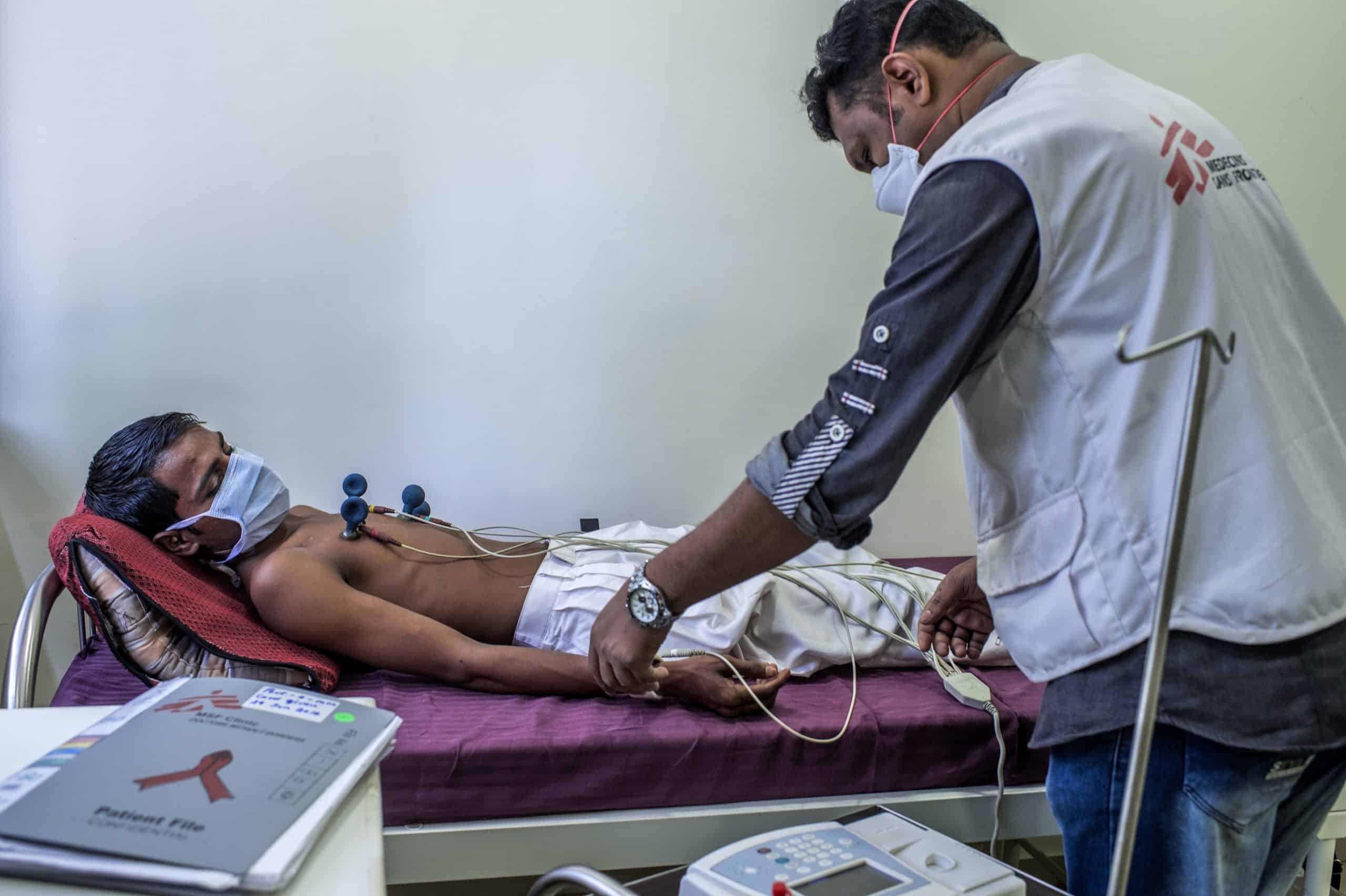
In India, as in most countries with high burden of DR-TB, the challenge is not just accessing the combination of Bedaquiline and Delamanid; it’s multifaceted. When these two medicines were introduced in 2013 and 2014 respectively, they were not immediately approved and were only available under compassionate use. As in most countries, it was only in 2016, a few years after MSF had started using these drugs, that the national TB programme introduced Bedaquiline under a pilot individual treatment for only six months in line with the existing WHO recommendations. Delamanid, however, was introduced in the country much later in 2019.
Before Bedaquiline and Delamanid, the drugs available to fight drug-resistant tuberculosis had enormous limitations. The side effects were debilitating, and the old drugs didn’t work in all patients. These new drugs brought hope to patients who had no other options for treatment. Patients with extensive drug resistance required not only Bedaquiline and Delamanid used together, but also that patients need to continue treatment beyond the 24-week WHO recommendation as one requires 4 effective drugs in a treatment regimen to treat DR-TB and such patients are left with very few sensitive drugs.
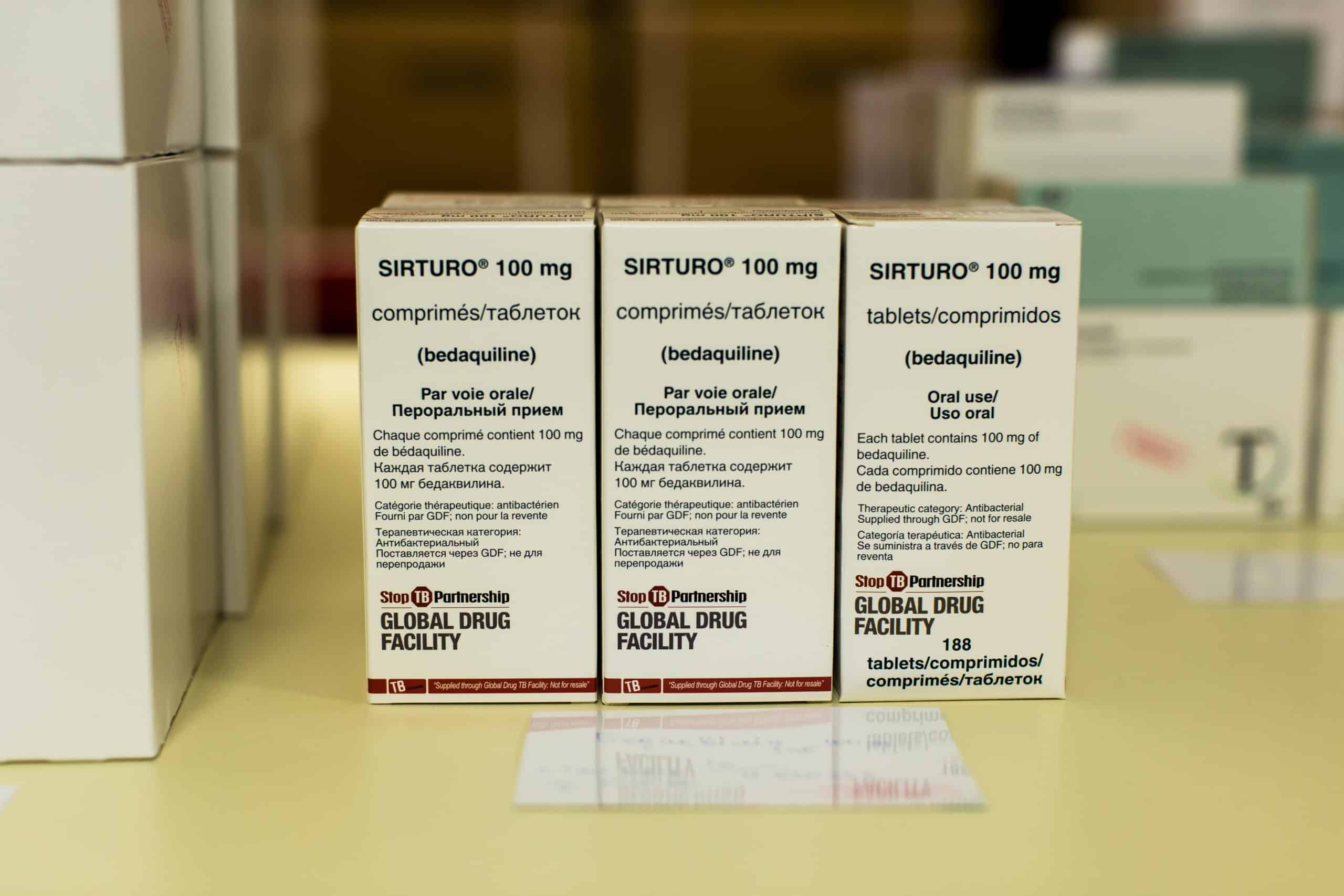
Unfortunately, due to a combination of challenges like limited scientific evidence on the safety and efficacy of this protocol, the authorities were then reluctant to support bulk importation and access through Global Drug Facility (GDF) donation: MSF had to resort to supporting patients to access drugs via individual importation route. These processes were complex, excruciatingly lengthy to obtain and outrageously expensive- as an example, the full treatment for one single patient taking Bedaquiline and Delaminid with imipenem costs a whopping 20,000 Euros and without imipenem 13.000 Euros is spent. However with GDF donation after approval from the national programme, this cost has gone down to 8.000 per patient.
These hurdles often led to heartbreaking scenarios, because for many patients, Bedaquiline and Delamanid was the last available DR-TB treatment option – and it wasn’t widely available.
The treatment initiatives by the National Institute of TB and Respiratory Diseases (NITRD) in Delhi and by MSF in Mumbai started about 2 to 3 years before the latest revisions to WHO guidelines. In this regard, India has been among the first countries to adopt this protocol, albeit as a pilot initiative reaching only a handful of patients. I am hopeful that this treatment would be soon included into national guidelines to be mainstreamed into routine use to save lives of thousands of DR-TB patients across the country and inspire uptake in other countries as well.
Getting the concomitant use of new drugs and their use beyond 24 weeks included in the WHO guidelines is indeed a success story but the war against TB remains a massive challenge. In most countries with high DR-TB, due to variety of reasons e.g. overcautious and conservative attitude of the physicians and national programmes, the lack of preparedness and capacities at different levels, there has been a slow uptake of much needed effective new drugs particularly in the initial phase. One must continue to challenge conventions and proactively advocate for access to the effective affordable diagnostics and treatments available to all by putting the patients’ interest at the core of their concern. Regulations and policies should ‘help facilitate access to life saving treatment not create barriers’ – as stated by the senior Indian MoH official while he approved MSF proposal for this regimen a year earlier than the WHO guidelines. If policy makers in most high TB burden have this kind of attitude, I am sure elimination of TB could become a reality in our lifetimes.











